Practical Seed Source Selection for Restoration Projects in an Urban Setting: Tallgrass Prairie, Serpentine Barrens, and Coastal Habitat Examples
by Danny J. Gustafson1, Angela C. Halfacre2, and
Roger C. Anderson3
1Department of Biology, The Citadel, Charleston, South Carolina 29409
2Department of Political Science, College of Charleston, Charleston, South Carolina 29242
3Department of Biological Sciences, Illinois State University, Campus Box 4120, Normal, Illinois 61709
Abstract
Anthropogenic activities have dramatically altered native plant communities through both habitat reduction and habitat fragmentation. Awareness of these changes has lead to an increased interest in restoring extirpated populations and augmenting remnant communities in urban, suburban, and agricultural landscapes. Ecological restoration frequently requires seeds of component species, and the choice of local, nonlocal, and cultivar seed sources could affect the success of a restoration project. In this article, we describe restoration projects conducted in tallgrass prairie, eastern serpentine barrens, and coastal South Carolina to illustrate practical advice on seed-source selection. We advocate the use of locally collected seed if available, but we acknowledge that nonlocal sources from similar ecological settings (via ecological matching), geographically local sources from different habitats, or unrestricted seed sources may be appropriate depending on the goals of the specific restoration project.
Key words: restoration ecology, local ecotype, Muhlenbergia sericea, sweetgrass, Gullah, community participation, tallgrass prairie, eastern serpentine barrens, coastal habitat
Introduction
When discussing plant community restoration, interested parties typically raise the following questions: What is a local seed source? How far can we go to collect plant material for our restoration project? Can we purchase seeds rather than collect them? Individuals interested in restoring, reestablishing, recreating, or augmenting a historical native plant community will likely be very interested in identifying and selecting local ecotypes. Seminal research and recent literature reviews have established the scientific justification for selecting local seed sources for restoration projects and offer general guidelines for select species and ecosystems (Clausen and Heisey 1958; Hufford and Mazer 2003; Joshi et al. 2001; Kawecki and Ebert 2004; Lesica and Allendorf 1999; Schaal and Leverich 2005; Linhart and Grant 1996; McKay et al. 2005; McMillan 1959; Montalvo et al. 1997; Packard and Mutel 1997; Rogers and Montalvo 2004). In this paper, we outline some commonsense considerations for seed-source selection when conducting plant community restoration projects within urban settings. To illustrate our points and provide real world examples, we share lessons learned about tallgrass prairies, serpentine barrens, and coastal habitats.
Defining Goals
From our experience and that of others, we argue that the first step to any successful restoration project-and, accordingly, to seed source selection-is to consider an array of goals and clearly define the context of the project and project priorities. We provide an overview of suggested questions to consider when planning and implementing a restoration project in urban areas and wildland-urban interfaces (Table 1). In the following section, we explore five of the more common goals for habitat restoration and reclamation. By examining these goals, we provide a range of options for restoration.
Goal One: Establishing a historical plant community is a challenging goal for anyone pursuing ecological restoration using historical local genotypes because in many regions there are few situations where organizers will find remnant populations with historical genotypes on-site.
Goal Two: Many individuals conducting ecological restoration focus on reestablishing native plant communities with local, but not necessarily historical, genotypes that would have once occupied the restoration site. Selection of plant material that has evolved under similar ecological conditions as the proposed restoration site should have genetic combinations (genotypes) that are more likely to be adapted to present ecological conditions than genotypes that have evolved under other ecological settings. For example, selecting seeds from a population in a wet habitat to restore a wetland community is most likely a better ecological match of plant material than selecting seeds of the same species from a dry habitat.
Goal Three: How should project organizers proceed if there are no local native sources or if local sites are too small to provide sufficient seeds for the entire restoration project? Without sufficient local native seed sources, restoration managers may choose to collect seeds of the desired species from different ecological settings or purchase seeds from a native plant seed supplier. When lacking sufficient seed sources, organizers have three possible approaches. Restoration professionals may decide to use the local seeds but may have to establish seed increase plots on-site and use a multiple-year approach to generate sufficient seeds for the entire project. Two potential negatives to this approach are: (a) multiple seasons needed to generate sufficient seeds may not fit within the time constraints of the funding source, and (b) danger of founder effects due to the small original gene pool used to establish the seed increase plots. Founder effects occur when a few individuals are used to establish a new population, with the resulting population containing only a fraction of the original genetic diversity. Negative effects associated with founder effects, genetic drift and potential inbreeding depression, can be reduced by establishing seed increase plots and/or restoration projects with seed from many individuals collected from across the original native plant community.
Additionally, restoration professionals may choose to collect seeds of target species from a relatively close geographic area but with less emphasis on ecological matching. For example, Cook County, Illinois, has approximately 67,000 acres of prairie, savanna, wetlands, and forest managed by the Cook County Forest Preserve District, and these natural areas could be potential seed sources for restoration efforts in the greater Chicago metropolitan area. The same can be said for Westchester County, New York, and its 39 county-owned natural areas, which could be seed sources for restoration projects in the greater New York City metropolitan area (see fcwc.org/directory/wcoppnc.htm).
Restoration professionals can also decide to purchase seed from a native plant seed producer, regardless of the origin of the original seed source. When using warm and cool season grasses in an urban project in the Northeast, participants can purchase cultivated varieties of these grasses that originated from Kansas, Nebraska, Oklahoma, and Illinois and are produced in large seed increase plots throughout the United States. This large-scale seed production results in relatively inexpensive high quality seed in sufficient quantities to do large-scale plantings. Some of these cultivated varieties have been developed as native forage crops and may not, however, be genetically and ecologically equivalent to native populations in this same region (Gustafson et al. 1999; 2004a; 2004b; Gustafson et al. 2005). Care should be taken in choosing seed because seed purchased from commercial sources may have a tendency to dominate restorations (Baer et al. 2004) as has been the case for Blackwell switchgrass under some circumstances (Schramm 1978).
There are naturally occurring patterns of adaptation across large geographic areas for species with fairly large species ranges (Gustafson et al. 2002; McMillan 1959) or a range of different ecological settings (Huff et al. 1998; Rice and Knapp 1998;). Rogers and Montalvo (2004, Table 10.3) nicely summarize 17 grass, 37 forb, 11 shrub, and 10 tree species studies that have investigated local adaptations or genetic differentiation in at least one state of the Forest Service Region 2 (Colorado, Kansas, Nebraska, South Dakota, Wyoming). While some of these native species do have extensive ranges, it may not be appropriate to assume that the patterns of local adaptation in these states accurately reflect selection dynamics in other regions of the country.
Goal Four: If the potential restoration site has been so dramatically altered (via strip mining, decommissioned landfills, phosphate mines, etc.) that few plants occur there, then the restoration practitioner may select native species that have the potential to grow on that site. This is not a restoration project per se, but more of a reclamation project where the goal is to establish a plant community on a highly disturbed site. Fresh Kills Landfill, on Staten Island, is a 2,200-acre landfill that officially closed in 2001 after it received debris from the World Trade Center. The City of New York is currently planning a large-scale reclamation/restoration of the Fresh Kills Landfill site to create a world-class park (see nycgovparks.org/sub_your_park/fresh_kills_park/html/fresh_kills_park.html). Reclamation of strip mines, decommissioned landfills, and brownfields offers an opportunity for restoration professionals to incorporate native plant species into large-scale reclamation projects. Depending on the duration and intensity of the original disturbance, the types of plant communities that can be established on these sites may provide valuable ecosystem functions that will enhance or create recreational opportunities, habitat for wildlife, and a focal point for the propagation of native plant species for use in these local urban landscapes.
Goal Five: The use of nonnative species does not constitute a native plant community restoration project. We chose to include the nonnative species option in our generic goals simply to establish the range of possibilities for seed-source selection. We do not, however, promote the use of nonnative species in any project that has the goal of restoring native plant communities.
In the following section we discuss three examples of habitat restoration or reestablishment of native plant communities in areas where they no longer exist. Using only locally adapted genotypes (ecotypes) to restore the community is desirable, yet often local native populations no longer exist in areas planned for restoration. In such situations, having clearly defined goals and a sound understanding of the ecology of the plant community—including species composition as well as disturbance dynamics—can help guide selection of plant materials and improve restoration project success.
Below we discuss three habitat examples with implications for the urban environment. In each case, we emphasize key elements of interest to restoration practitioners, and we provide an overview and a project example. In the case of the coastal habitat, we focus our efforts on a native species used for restoration by long-term residents in the region. Since this case has added socioeconomic relevance, we provide more detail of the context of these restoration efforts.
Case One: Tallgrass Prairies
Click image to enlarge


Figure 1: Top: Weston Cemetery Prairie, McLean County, Illinois, in the summer of 1977. This remnant five-acre pioneer cemetery prairie is surrounded on three sides by row crop agriculture and a railroad right-of-way leading to the grain elevator in the background. Fire management is used to reduce woody cover and promote species-rich forbs and grasses. Bottom: Railroad prairie located 16 miles west of Madison, Wisconsin, taken in 1964. These long, linear-shaped remnant prairies have been historically maintained by fire management of woody species by the railroad companies, however modern vegetation management uses herbicide. Removal of the natural fire regime is resulting in encroachment by fire intolerant woody species and the loss of fire dependent prairie species. (Photos by Roger C. Anderson)
Preservation and conservation of native grasslands has steadily increased during the last three and half decades, although efforts to restore degraded or extirpated communities are hampered by the scarcity of remnant sites. Remnant North American tallgrass prairies, for example, currently occupy < 0.01% of their historical range (Packard and Mutel 1997), with many of the highest quality remaining remnants as small pioneer cemeteries and linear-shaped railroad right-of-ways (Figure 1). Conversion of the nutrient rich native grasslands into row crop agriculture has reduced the size of the remaining grasslands and increased the distance between native sites beyond many species dispersal distances. Organizations and private individuals have taken an active role in restoring native communities throughout North America, with many scientists and restorationists agreeing that matching ecologically appropriate genotypes to restoration site conditions will increase the likelihood of a successful project. The problem lies in the fact that there are very few remaining remnant grasslands and that many of them are very small (≤ 5 acres).
Project: A local elementary school in central Illinois wanted to restore a section of tallgrass prairie along the public bicycle path behind the school. The Freedom Prairie, as it is known, is located south of Colleen Hoose Elementary School, along Constitution Trail, in Normal Illinois. Restoration of this small (10 meters by 50 meters) prairie began in the spring of 1990 and was sponsored by the John Wesley Powell Chapter of the Audubon Society. McLean County Illinois once had 683,136 acres of tallgrass prairie; by the time of this restoration project, only one pioneer cemetery prairie (~5 acres) state-protected remnant was left (Anderson 2006). There were, however, several native prairie communities along railroad right-of-ways and several restored prairies established by The Nature Conservancy, local universities, and civic groups interested in promoting prairie and savanna ecosystems. The goal of this school's project was to establish a small-scale historical plant community that included local ecotypes (Goal #2). The initial planting of the Freedom Prairie was accomplished by hand broadcasting native warm season grass seed purchased from commercial grower and elementary school children planting native prairie plant root stocks obtained from the Illinois Department of Natural Resources State Nursery, Mason County, Illinois. As with many restoration projects, one can increase plant species diversity by planting seeds of additional native species collected from local remnant sites. If the project had been to establish a tallgrass prairie on a 100-acre parcel of land taken out of row crop or pasture production, then the planning and implementation of the project would have been much more complex.
Case Two: Eastern Serpentine Barrens
The eastern serpentine barrens are historical fire-dependent grasslands of the mid-Atlantic region of North America. These communities are characterized by unique plant assemblages, globally endangered barrens aster (Symphyotrichum depauperatum) (Figure 2), hairy chickweed (Cerastium arvense ssp. velutinum var. villosum), and shallow soils with high levels of magnesium, nickel, and chromium in concert with low phosphorus, calcium, and potassium (Brooks 1987; Gustafson et al. 2003; Gustafson and Casper 2004; Gustafson and Latham 2005; Latham 1993). It is believed that eastern serpentine grasslands once covered approximately 100,000 acres of the mid-Atlantic prior to European settlement, but currently there are fewer than 26 serpentine sites ≥ 5 acres from Georgia to Vermont. Removal of the natural fire dynamic and encroachment by urban development have contributed to the loss of these barrens (Latham 1993; Tyndall and Hull 1999). The unique flora of serpentine barrens is a consequence of the origin of serpentine soil, soil chemical composition, and the fire dynamic. Serpentine soil conditions are often associated with edaphic ecotypic variation or locally adapted genotypes that have evolved under these strong selective pressures of the serpentine soils (Brooks 1987).
Project: Restoration of a serpentine barren in the Philadelphia metropolitan area, Pennsylvania.
The urban expansion within the greater Philadelphia metropolitan area has destroyed many small remnant serpentine barrens. Most of these lost sites are in such poor condition that the locals do not even know that they have a unique serpentine plant community nearby (Latham personal communication). Restoration of these urban serpentine barrens typically requires removing tree species to reopen the canopy, replanting dominant grasses that are a significant component of this community, and reintroducing to the site rare species like the barrens aster and hairy chickweed. Given that there are few remaining eastern serpentine barrens and these sites may not be in close geographic proximity to the restoration site, restoration professionals may choose to purchase the grass seed from native plant suppliers and only field collect for select species like the barrens aster (Goal #3). The warm season grasses—such as big bluestem (Andropogon gerardii), Indian grass (Sorghastrum nutans), little bluestem (Schizachyrium scoparium), and prairie dropseed (Sporobolus heterolepis)—are significant components of the serpentine barrens; they have been shown to contribute to plant community structure through plant-soil feedback interactions, and they provide much of the biomass fuel needed to carry a fire, which is an important dynamic of the eastern serpentine barrens (Castelli and Casper 2003; Casper and Castelli 2007; Gustafson and Casper 2004; Latham 1993). Seeds of these warm season grasses can be purchased from native seed vendors in Pennsylvania and New Jersey, but to the best of our knowledge none of these vendors specifically collects and propagates serpentine barrens collections. In this case, restoration professionals would have to decide if they want to purchase grass seeds originally from those states (Pennsylvania and New Jersey), purchase seeds from Midwestern or Plains states (Illinois, Missouri, Iowa, Kansas, Nebraska, Oklahoma), or if they want to establish on-site seed increase plots using only serpentine-collected plant material.
Case Three: Coastal Habitat and Sweetgrass
Coastal South Carolina is characterized by coastal plains, expansive estuaries, barrier islands, and back barrier (hummock) islands (Porcher and Rayner 2001; SCDNR). This region is known for its heat, humidity, mosquitoes, and hurricanes; however, residents commonly focus on the appealing climate for most of the year, natural beauty, and sociocultural distinctiveness (Halfacre et al. 2007). African-Americans living in the region, descendants of enslaved Africans, have maintained cultural traditions that were forged through the forced relocation of these peoples (NPS 2001). Gullah culture includes unique speech, religious beliefs and practices, family social units, music, dance, storytelling, arts and craftsmanship, and use of coastal resources (Crook et al. 2003; Pollitzer 1999). The terms Gullah and Geechee are often both used to describe similar cultures, but in South Carolina, Gullah is used to a greater extent than Geechee. Sweetgrass basketry is one of the cultural traditions preserved along the Gullah/Geechee coastline (NPS 2001).
Basketry was first introduced to the Carolina coast in the late seventeenth century (Rosengarten 1986), and sweetgrass basket making became increasingly important during the development of the tourism industry during the early twentieth century (Coakley 2006). Basket-making skills were carried over from slaves' homelands and were quickly adapted to the raw materials available in coastal South Carolina. The signature plant material used to make sweetgrass baskets comes from the perennial grass Muhlenbergia sericea (synonyms: Muhlenbergia filipes and Muhlenbergia capillaris var. filipes), which occurs in sandy maritime habitats on barrier islands and coastal woodlands along the southeastern and gulf coasts of the United States (Gustafson and Peterson 2007; Peterson 2003; Porcher and Rayner 2001; Radford et al. 1968).
Historically, the basket was used for fanning rice on plantations; after emancipation, the basket makers produced containers for storing food and other household items. Local residents used these baskets for day-to-day agricultural and household purposes; they were objects of necessity. However, around the turn of the twentieth century, a group of black Mount Pleasant families began mass-producing more intricate "show baskets" (Figure 3) made from sweetgrass and bound with strips of palmetto leaf (Rosengarten 1986). Extirpation of historical M. sericea populations and urban development along the coast have resulted in fewer collectable populations, forcing basket makers to purchase or travel several hundred miles to collect sufficient plant material (Burke et al. 2003). Charleston's growth as a tourist destination and the associated rapid expansion of suburban and exurban residential development have both created a wider market for these baskets and threatened the basket makers' access to resources (Allen 2002; Hurley and Halfacre in press; World Travel and Tourism Council 2001).
Historically, basket makers and their families have had tacit arrangements for collecting plant materials on private property, but changes in ownership and suburbanization have altered these arrangements and diminished once readily available supplies. These stakeholders often express an interest in plants from local "wild" populations, citing qualitative attributes. These desires call attention to the differences between sweetgrass local ecological adaptations and the cultural preferences rendered by the basket makers (Halfacre et al. draft). Persistent public attention has enhanced the artistic, cultural, and monetary values of sweetgrass baskets, and it is an important source of supplemental income for black artisans (Allen 2002; Coakley 2006; Derby 1980; Hart et al. 2004).
Click image to enlarge


Figure 4: Characteristic Muhlenbergia sericea habitat on front barrier (Top: Dewee's Island) and back barrier (Bottom: Apron Island) islands in Charleston County, South Carolina. This species can occur in the interdunal troughs between the established dune communities, areas without significant woody vegetation, and in the ecotone between the salt marsh community and maritime forest. The typical flowering period for Muhlenbergia sericea along the South Carolina coast is from the middle of October through November. (Photo by Danny J. Gustafson)
Project: To establish a plant material source for sweetgrass basket makers in Mount Pleasant and Charleston, South Carolina.
Sweetgrass naturally occurs along barrier islands and the mainland juxtaposed between salt marshes and maritime forest from South Carolina to Texas (Pinson 1971; Ohlant 1992; Peterson 2003). While we can often find small populations on many barrier islands along coastal South Carolina, these populations typically have fewer than 20 mature individuals (Figure 4). In addition to not having abundant numbers of individuals within a population, the sweetgrass basket makers have indicated that they are no longer able to access sufficient areas to collect material to make their baskets (Hart et al. 2004).
Muhlenbergia sericea is recognized as a distinct species and not a variety of the more widely distributed M. capillaris, based on anatomical, cytological, genetic, and ecological data (Gustafson and Peterson 2007; Peterson 2003). The Citadel Plant Ecology Laboratory (CPEL) has established common garden experiments in the greenhouse and on a back barrier island (Apron Island) in Charleston County, where we looked at plant performance relative to origin of the original seed source (Charleston County, South Carolina, and Kennedy County, Texas). The South Carolina plants had lower flowering rates in the greenhouse and higher survivorship rates on Apron Island than plants originally from Texas (Figure 5). Genetic research with material from these same populations indicated that the Texas plants were genetically different from the South Carolina plants, and we have thus identified ecotypic variation between plants collected from the eastern- and western-most sections of the species range. From a practical restoration and conservation perspective, it is not realistic to think that a sweetgrass restoration project in the Carolinas would use plant material from as far away as Texas, but we have shown that ecotypic variation does occur with this species.
Click image to enlarge
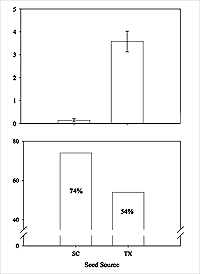
Figure 5: Top: In a 6–month greenhouse common garden, 34 of 50 plants from Texas flowered while 1 out of 50 South Carolina plants flowered (χ2 = 60.14, d.f. = 1, P < 0.0001). These same plants were transplanted to Apron Island in October 2005, and plant survival was recorded in May 2006. Bottom: South Carolina plants had higher survivorship than plants from Texas.
The next step in providing collectable populations of sweetgrass for the local sweetgrass basket makers is to determine to what extent ecotypic variation occurs in native populations of M. sericea along the historical range of the Gullah corridor. In addition to plant ecological research, researchers at the College of Charleston and Clemson University are presently collecting data to understand better the historical and cultural resources and land use present in the Gullah/Geechee Heritage Corridor, with a focus on the Mount Pleasant, South Carolina area. By approaching the issue of collectable populations of sweetgrass for the basket makers of the Gullah Corridor, we are addressing modern, complex problems from a multidisciplinary perspective.
Contrary to what was the case in the tallgrass prairie and serpentine barrens restoration projects, there are no commercially available seed sources of a cultivated variety of M. sericea. There is, however, a growing ornamental container sweetgrass industry that is planting both M. sericea and M. capillaris in urban settings throughout the southeastern U.S. It is not clear what effect these ornamental landscape plants will have on the native genotypes, for example, if nonlocal genes from these landscape plantings will swamp the local native populations and what effect that will have on population persistence, or if these landscape plants will provide sufficiently high quality and quantity of plant material for sweetgrass basket makers. What is clear is that the southeastern United States is one of the fastest growing regions in the nation (U.S. Census 2000 and 2005), and urban expansion will likely continue to diminish the accessibility of historical sources to sweetgrass basket makers.
There is a growing need to establish collectable populations of M. sericea for Gullah communities along the Gullah/Geechee National Heritage Corridor. Attention should also be paid to reducing the destruction of existing native populations and to diminishing the impact of horticultural plantings of nonnative container plants as a result of urbanization. In this situation, establishing community gardens or planting with appropriate plant material should be the restoration/reclamation goal, however it is too early to know if the appropriate plant material is from the historical Gullah Corridor (Goal # 1), a coastal Carolina ecotype (Goal #2), or some source from Florida or the Gulf Coast (Goal #3).
Conclusion
In this paper, we have cited specific examples of ecological restoration projects that we have experienced firsthand in the Midwest, Northeast, and Southeastern U.S. We promote the use of locally collected plant material if available, but we have faced situations where matching ecologically the donor habitat with the restoration site was simply not possible. Under such circumstances, purchasing seeds of the desired species from native plant suppliers allowed us to use material from the plant adaptation regions (PAR). PAR combines USDA plant hardiness zones with the ecoregion system (epa.gov/wed/pages/ecoregions.htm) commonly used by nongovernmental organizations (NGOs) like The Nature Conservancy, and is supported by plant material test results conducted by academic and governmental agencies (Vogel et al. 2005). There are restoration situations in which historical ecotypes no longer exist—local populations having been long extirpated due to anthropogenic activities—and there are no commercially available seed sources originally from the same PAR. In such situations, we would advocate using nonlocal seed sources of the desired native species: Since a species range can be geographically and ecologically broad, it is better to plant a native plant community within the historical range of the component species rather than use nonnative species or fail to conduct any restoration activities on degraded or damaged lands.
Acknowledgments
We would like the acknowledge contributions to the various projects highlighted in this paper, specifically Drs. David J. Gibson, Daniel L. Nickrent, Brenda B. Casper, Roger Latham, Marianne Burke, and Carol Preston. We thank Drs. Joel Gramling and Patrick Hurley for thoughtful comments that improved this manuscript. We would also like to thank the Illinois Department of Natural Resources, South Carolina Department of Natural Resources, South Carolina Sea Grant, NOAA, College of Charleston, The Citadel Foundation, The Nature Conservancy, and The Citadel for their financial support. We appreciate the willingness of residents to share their perceptions and experiences with us.


