Japanese stilt grass (Microstegium vimineum), a nonnative invasive grass, provides alternative habitat for native frogs in a suburban forest
by Christopher Nagy1,2, Seth Aschen¹, Rod Christie1, and Mark Weckel1,2
1Mianus River Gorge Preserve, 167 Mianus River Road, Bedford, NY 10506
2American Museum of Natural History, Central Park West, New York, NY 10024
Abstract
Japanese stilt grass (Microstegium vimineum) is an invasive grass in the eastern and midwestern United States. It tolerates a wide range of light and moisture conditions and has readily replaced native herbaceous vegetation in many areas. Despite its detrimental effects, Japanese stilt grass does provide some benefit, serving as habitat for ground amphibians such as frogs and toads (anurans) in areas where populations of white-tailed deer (Odocoileus virginianus) have depleted native herbaceous cover. We investigated relative abundances of common anuran species both inside and outside of a stilt grass invasion front in a Northeastern mixed hardwood forest. We used pitfall trap arrays to sample anuran species during the summers of 2006 and 2007 and we captured four species: wood frog (Rana sylvatica); pickerel frog (Lithobates palustris); spring peeper (Pseudacris crucifer); and American toad (Bufo americanus). We captured more individuals from each of these species in stilt grass plots. Too few spring peepers were captured for analysis, so we modeled the captures of the three remaining species—wood frog, pickerel frog, and American toad—with negative binomial regression against stilt grass presence/absence, soil moisture, soil temperature, and relative humidity. We compared models containing these parameters using AIC (Akaike's Information Criterion), a common information criterion used in model selection. All three species selected stilt grass plots, but appeared to do so for different reasons. Pickerel and wood frogs seemed to select primarily areas of high soil moisture, which was consistently greater in stilt grass plots. American toads selected stilt grass areas and areas of high humidity, though humidity did not vary according to stilt grass presence or absence. For all three species, stilt grass seemed to provide habitat value beyond any causal or correlative relationship with microclimate. These results suggest that some invasive species of herbaceous cover provide alternative habitat for native wildlife in degraded communities. Managers need to consider the effect on wildlife when considering removal of invasives, particularly when there is little native habitat and when removal would be destructive.
Keywords: Japanese stilt grass (Microstegium vimineum), wood frog (Rana sylvatica), pickerel frog (Lithobates palustris), American toad (Bufo americanus), invasive species management, Mianus River Gorge.
Introduction
The rapid proliferation of exotic invasive species is one of the most serious threats to native biodiversity (Binggeli 1996; Vitousek et al. 1997; Chornesky and Randall 2003). Exotic plants in particular are typically regarded as undesirable community elements capable of outcompeting native species (D'Antonio and Mahall 1991; Hamilton et al. 1999), interrupting mutualisms (Kearns et al. 1998; Stinson et al. 2006), influencing rates of chemical cycling (Ehrenfeld 2003), and influencing successional trends (Stromayer and Warren 1997). The removal of populations of invasive species is therefore often the primary objective—and primary drain of resources—of many management and restoration programs (D'Antonio and Meyerson 2002; Ewel and Putz 2004). However, there are circumstances in which invasive plant species may provide habitat or food for native fauna (Hunter et al. 1988; Chen 2001; Zavaleta et al. 2001). If native species are inhibited or completely usurped by nonnative species, managers face the choice of removing invasives (effectively destroying habitat) or allowing them to remain at the risk of the aforementioned impacts (D'Antonio and Meyerson 2002). On the surface, this dichotomy appears to pit single-species management against a more community- or ecosystem-focused approach; however, it has been suggested that there may be a use for invasive flora in the restoration of degraded sights (Ewel and Putz 2004; Lamb 1998; D'Antonio and Meyerson 2002). Managers should also bear in mind the potential role of nonnative flora as habitat when contemplating a comprehensive restoration plan where such species provide the only available habitat.
In the northeastern United States, sustained, high-density populations of white-tailed deer (Odocoileus virginianus) have eliminated or reduced native herbaceous and woody communities (Anderson and Loucks 1979; Tilghman 1989; Stromayer and Warren 1997; Waller and Alverson 1997). Unequal deer browsing on native rather than invasive plants facilitates the establishment and proliferation of invasive species while severely inhibiting the growth of native species (Rooney and Waller 2003). Little research has been done to examine the potential role of invasive plant species as a surrogate component of wildlife habitat (e.g., Hunter et al. 1988). In this exploratory study, we investigated the impact of Japanese stilt grass (Microstegium vimineum, hereafter stilt grass), on terrestrial frog and toad species (anurans) in a suburban Northeastern nature preserve.
Stilt grass is native to southeastern Asia (China, Japan, Korea, Malaysia) and India, and was first found in the United States in 1919 (Barden 1987). It is tolerant of a range of soil and light conditions (Cole and Weltzin 2004), can form dense monocultures on the forest floor (Barden 1987; Leicht et al. 2005), and represents a new habitat component to Northeastern forests. Stilt grass appears to replace native herbaceous species readily in deer-modified environments (Rutberg et al. 2004), yet little has been done to explore how its structural or microclimate conditions might make it a potentially suitable habitat for small fauna. We compared species-specific captures of one toad and three frog species, American toad (Bufo americanus), wood frog (Rana sylvatica), pickerel frog (Lithobates palustris), and spring peeper (Pseudacris crucifer), on opposite sides of a stilt grass invasion front. Specifically, we sought to model captures as both a function of stilt grass presence/absence and of microclimate variables. Soil moisture (Dole 1967; Lillywhite and Licht 1974; Tracy 1976; Wyman 1988; Vonesh 2001), soil temperature (Brattstrom 1963; Tracy 1976; Duellman and Trueb 1994), and relative humidity at ground level (Duellman and Trueb 1994) were selected as candidate model parameters because of their known effect on habitat use by these species.
Methods
Study Site and Field Methods
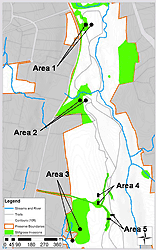
The study was conducted at the Mianus River Gorge Preserve (MRGP), a 305-hectare nature preserve in Westchester County, NY. The MRGP consists of old-growth eastern hemlock (Tsuga canadensis) and mid-succession hardwood forests surrounded by suburban development. The hardwood sections of the Preserve were croplands until about 70 to 100 years ago. The preserve has a high deer density (> 22 deer/km²; personal communication, Vector Ecology Lab, Fordham University) typical of many northeastern suburbs (Porter et al. 1991; Rondeau and Conrad 2003). Both the hardwood and hemlock areas have exhibited sharp declines in their woody understory (Weckel et al. 2006) and herbaceous diversity (unpublished data from MRGP), though the hemlock sections remain free of many invasive species. In 2006, sampling was limited to three large (> 1000 m²), well-established stilt grass populations (Areas 1, 2, and 3) within the hardwood forest; stilt grass has yet or is unable to invade the old-growth hemlock stands. Since stilt grass tends to colonize moist areas, we tried to distribute our paired study plots across a range of moisture gradients. Area 1 was located in a lowland moist forest patch (i.e., the bottom of a slope); Area 2 was located in a moderately moist forest patch along a slight slope; and Area 3 was located in a lowland wet meadow. Two smaller populations (approx. 225 m²; Areas 4 and 5) were added in 2007 to increase sampling effort in drier upland areas at the crest of the Preserve's gorge (Figure 1).
In each area, we established paired trap arrays: one array in the area covered by stilt grass and another nearby but past the stilt grass front. "S" arrays consisted of > 75% stilt grass cover and "N" arrays contained no stilt grass, assessed by visually estimating percent cover within 3 1m² quadrats when arrays were first placed. Trap arrays consisted of pitfall traps made from buried 5-gallon buckets and drift fencing made of plastic silt fencing material. A large trap array (Areas 1–3) consisted of a central pitfall bucket with three 5-meter fences leading away from it, with approximately 120° between each fence. At the far end of each fence, we buried another pitfall trap. The circular perimeter of a large array covered an area of 78.5m². Small trap arrays (Area 4 and 5) consisted of a single 5-meter drift fence with two 5-gallon buckets on each end. Typical for this forest, "N" arrays also had < 10% native or other invasive herbaceous cover; in most cases the ground cover was bare earth or leaf litter. The exact locations of the trap arrays in both treatments were dictated largely by finding level areas large enough to fit the arrays. Depending on the particular plot, the individual trap arrays in each pair ranged approximately 25 to 100 meters away from each other, although the "N" arrays were never more than 25 meters away from the edge of the stilt grass invasion. When not in use, pitfall traps could be covered with lids held in place with weights.
Areas 1 through 3 were sampled twice per week from June 23, 2006 to September 1, 2006 and from July 7, 2007 to August 31, 2007. Areas 4 and 5 were sampled only in 2007. Traps were opened in the late afternoon and checked the following morning. Each large trap array was sampled 34 times over the two summers and each small array was sampled 15 times in 2007 (936 trap nights total). Areas 1, 2, and 3 (large arrays) were sampled 136 trap nights each (78 trap nights per treatment) and Areas 4 and 5 (small arrays) were sampled for 30 trap nights total (15 trap nights per treatment). We recorded the species and number of all anurans caught. Soil temperature (C), percentage soil moisture, and ground level relative humidity (collectively referred to as microclimate variables) were measured at three random points within each array on each trap visit. Soil temperature was measured using a Taylor soil thermometer and soil moisture was measured using a Kelway Soil Tester, both at a depth of 10 centimeters. Relative humidity was measured with a Kestrel 3000 Pocket Weather Station at ground level. All captured animals were released immediately, outside the trap array circle (i.e., a distance greater than 5 meters away). We did not mark captured animals.
Analysis
Throughout the analysis we used the model-selection or information-theoretic approach discussed by Burnham and Anderson (2002) instead of the more traditional Neyman-Pearson hypothesis-testing approach. In brief, the Burnham-Anderson approach compares a set of models, each representing an a priori hypothesis, to determine which is closer to a hypothetical model that encompasses all of reality, i.e., one that perfectly models the dependant variable in all instances. Models are ranked using Akaike's Information Criterion (AIC), which penalizes the maximum likelihood of a model proportional to the number of parameters in the model. The rankings are relative and contingent on a single data set since the "perfect" model cannot be known (i.e., AIC scores cannot be compared across data sets). As well, the predetermined hypotheses must be well researched and relevant to the question at hand, as AIC will still identify a "best" model even from a set of universally poor models. The advantages and general differences of an information-theoretic approach versus traditional hypothesis testing were discussed by Anderson et al. (2000). One should not mix hypothesis testing (i.e., significance tests) with AIC or other information-theoretic approaches.
Because each paired trap array had equal trap nights, we were able to model the daily number of captures in "N" versus "S" plots rather than using the rate of captures per trap night. Daily capture observations from both summers were modeled as functions of stilt grass presence/absence and soil moisture, soil temperature, and relative humidity using negative binomial regression. This form of regression is based on the negative binomial distribution, which has a right-skewed frequency distribution with an excess of zeroes. Trapping and other count-based data typically have such a distribution (Cameron and Trivedi 1998). We evaluated a set of 11 candidate models to determine which of the four variables would best model species-specific captures using AICc (AIC adjusted for small sample sizes). Model fit for each species was assessed via examination of the Pearson chi-square ratio of the global models (the model consisting of all candidate parameters). This ratio is simply the chi-square statistic divided by degrees of freedom; numbers close to one indicate general model fit. If a global model fits the data suitably, then any subset model will have a similar fit (Burnham and Anderson 2002).
AICc was used instead of QAICc (AICc corrected for overdispersion; Richards 2008) since overdispersion was accounted for by negative binomial modeling. Akaike weights were calculated for each model to determine which of the candidate models best explained the data. Individual parameter weights were used to rank microclimate variables for each species. Stilt grass presence/absence could not be ranked in this way because it was included in a greater proportion of the model set than other variables.
Inference from the single best model may not yield the most precise estimates unless the best-fit model is vastly superior to the others. If a number of models are supported it is preferable to draw inferences from these models rather than the single best model. In order to calculate the average estimates of each parameter, subsets consisting of supported models were made. We defined "supported" by calculating relative likelihood of all models and including those models with a relative likelihood > 1/8 in the subset for each species. Model-averaged coefficients and unconditional standard errors were calculated using these subsets and their recalculated Akaike weights (Burnham and Anderson 2002).
Results
Four anuran species, wood frog, pickerel frog, spring peeper, and American toad, were captured during both seasons, more often in "S" arrays than "N" arrays. Specifically, we caught 24 wood frogs inside stilt grass arrays and 15 outside them, 46 pickerel frogs inside and 18 outside, 10 spring peepers inside and 5 outside, and 47 American toads inside and 30 outside.
All microclimate conditions remained fairly stable in each area throughout each season. Soil moisture appeared to be positively associated with stilt grass presence (48.98 + 1.77% vs. 37.48 + 1.60% [mean + SE]). Soil temperature (21.28 + 0.12°C vs. 20.70 + 0.13°C) and humidity (88.48 + 0.65% vs. 85.78 + 0.92%) were higher in S plots, although the biological effect of these small differences is arguable.
We constructed models for three of four species; not enough spring peepers were trapped to provide sufficient data to build models for this species. Our model sets for each species consisted of: four 1-parameter models of each main effect (stilt grass presence/absence [Stilt], soil moisture [SoilM], soil temperature [SoilT], and relative humidity [Hum]); three 2-parameter models consisting of stiltgrass and each main efect; three 3-parameter models with interaction terms crossing stilt grass and each microclimate variable; and a global model containing all main effects and the three interactions. Global models for all species sufficiently fit a negative binomial distribution (Pearson chi-square ratio of global models = 0.99, 1.05, and 1.04 for pickerel frog, wood frog, and American toad, respectively).
Parameter estimates for pickerel frog models indicated that this species selected stilt grass presence and greater soil moisture as evidenced by the most supported model (Stilt + SoilM: ΔAICc = 0, w = 0.4749; Table 1), with some evidence of a negative interaction between the two (Stilt*SoilM: β = -1.950 ±1.799) (Table 4). Parameter estimates for wood frog models indicated that this species also selected areas of high soil moisture (SoilM: ΔAICc = 0, w = 0.2769) and were caught more often in stilt grass ([Stilt*SoilM] + Stilt+SoilM: ΔAICc = 0.0594; Table 2), although these two variables were not as singularly important as with pickerel frogs. There was, as with pickerel frogs, evidence of a negative interaction between stilt grass percent cover and soil moisture ([Stilt*SoilM]: β = -4.013 ±2.228; Table 4). In addition, soil moisture had the highest parameter weights for pickerel frog models and wood frog models (Table 5). Parameter estimates for American toad models indicated that this species selected areas with high relative humidity and stilt grass percent cover (Stilt+Hum: ΔAICc =0, w=0.3276) (Table 3). However, evidence of a negative interaction between stilt grass percent cover and relative humidity was found (Stilt percent cover: β= -0.077±1.269) (Table 4). Humidity had the highest parameter weight for American toad models (Table 5).
Discussion
Our objective in this study was to investigate the possibility that common anuran species use stilt grass in place of native herbaceous habitat that has been diminished by high herbivore densities. We hypothesized that stilt grass could provide favorable habitat by altering soil moisture or ground level humidity by limiting evaporative water loss compared to the mostly bare leaf litter of adjacent, noninvaded areas. The dense ground cover provided by stilt grass might also alter temperature, either by insulating soils or blocking sunlight. These altered conditions might provide favorable conditions for terrestrial anurans.
An assumption of these hypotheses was that the observed microclimate trends were the result of stilt grass presence/absence, not preexisting microclimate patterns that facilitated stilt grass colonization. Stilt grass is thought to more readily colonize moister areas (Gibson et al. 2002) and most of the sizable stilt grass invasion sites in the MRGP are adjacent to wetland areas. However, we arranged our plots to take into account a broad spectrum of moisture gradients: Two of our six plots were established in drier, upland areas of the Preserve and one plot was established along a slope, yet similar microclimate patterns were observed across all of our plots. Furthermore, trap arrays were paired within particular areas, thus, we have some confidence that if microclimate conditions in S-plots reflect preexisting conditions, then the corresponding N-plots would closely resemble their paired S-plots. Ultimately only soil moisture proved to vary measurably between invaded and non-invaded areas. Ehrenfeld et al. (2001) also observed this pattern and concluded that the thick thatch formed by stilt grass litter retains more moisture compared to native oak and common exotic litter.
For each species, a single microclimate variable emerged as the most important. For pickerel frogs and wood frogs this key variable was soil moisture and for American toads, relative humidity at ground level. However, for all species, models that also included stilt grass as a main effect and those that included a stilt grass interaction received substantial support, indicating that stilt grass provides some habitat value beyond its effect on any supported microclimate variable. Possibilities include greater cover (Seebacher and Alford 1999; Block and Morrison 1998) and/or macro-invertebrate (i.e., prey) abundance in stilt grass. Nonnative earthworms (present throughout the MRGP) have been found in greater abundance in soils colonized by exotic species (Kourtev et al. 1998, 1999) and are often preyed upon by anurans (Behler and King 1998). Maerz et al. (2005) found earthworms to be an important—albeit inconsistent—salamander prey item, often only utilized on rainy nights. It is possible that earthworm availability is more consistent in the wetter, exotic stilt grass habitat. In addition, while exotic earthworms are thought to reduce litter depth (Hendriksen 1990; Liu and Zou 2002) to the detriment of macro-invertebrates (i.e., native prey species; Migge 2001), stilt grass monocultures tend to generate thick litter layers (Gibson et al. 2002; Leicht et al. 2005), which decompose relatively slowly compared to native oak and other exotic litter (Ehrenfeld et al. 2001). If stilt grass provides a large amount of litter to native invertebrates and is simultaneously associated with high earthworm abundance, this could result in a large difference in anuran prey abundances between invaded and noninvaded sites.
The negative interaction between soil moisture and stilt grass for pickerel and wood frogs suggest that these species congregate in stilt grass patches for the greater relative moisture but do not need to do so on overall wetter days (e.g., following rainfall). Following precipitation, frogs would be equally or less likely to be found in stilt grass as not. The stronger support for the Stilt*SoilM interaction model over the simpler Stilt + SoilM main effects model in wood frogs may indicate that this "interaction threshold" occurs at a lower moisture level, and hence more frequently, for wood frogs than pickerel frogs. If stilt grass does in fact have a causal effect upon soil moisture, as suggested by Ehrenfeld et al. (2001), the observed patterns suggest that the primary reason for the greater captures of wood and pickerel frogs in stilt grass is that stilt grass increases local soil moisture. In both frog species, selection for locations with high soil moisture and dense ground vegetation (e.g., wet meadows) has been observed frequently (DeGraaf and Rudis 1983; Wyman 1988; Redmer and Meirzwa 1994; Baldwin et al. 2006).
American toads appeared to select stilt grass patches in all supported main effect models, while in the supported interaction model, there was a negative stilt grass effect but a positive interaction with humidity (which actually predicts greater toad captures in stilt grass within 0.8–100% humidity). Humidity has been shown to affect toad movement and distribution (Ewert 1969; Fitzgerald and Bider 1974), so its association with toad captures in this study is not surprising. American toads were caught more often in areas of high relative humidity; however, the model average coefficient for stilt grass was -0.077 ± 1.269, which reflected differences in stilt grass effect depending on the model used. Because relative humidity did not appear to vary with stilt grass presence, we believe that American toads select stilt grass for reasons other than microclimate (e.g., structural habitat or prey abundance). It is reasonable that soil moisture, the only microclimate variable that did vary across stilt grass plots, would not affect the relatively terrestrial toads as much as it did the pickerel and wood frogs.
An interesting extension of the above would be to relate species-specific captures to local rainfall data. However, the pitfall traps were open approximately 12 to 24 hours; a particular frog could have entered the trap at any time since the traps were opened the previous day or evening. Thus, to properly compare anuran activity to rainfall information the exact timing of the precipitation and each frog capture is needed. Similarly, plot-specific rainfall data (as opposed to data at the town or county level) would also be necessary, since a small rain shower may not hit all of the plots but may still affect anuran activity. We attempted a cursory comparison between captures and rainfall data recorded at the town level, but there were only 16 days with recorded rainfall greater than 0.1 centimeters. This made meaningful comparisons between days of low and high precipitation impossible.
Overall, the two frog species seemed to select primarily greater soil moisture, which was provided by stilt grass patches on most days. On days with greater soil moisture, there was some support from the interaction models that the two frog species are not constrained to the stilt grass patches. Alternately, American toads were caught more often on humid days, but selected stilt grass either independently of or increasingly with greater relative humidity.
This research contains important caveats. First, we acknowledge the limitation of not employing a mark-recapture framework in this trapping study. However, we have confidence that identical arrays of pitfall traps would have similar capture (i.e., detection) rates across "N" and "S" plots, and therefore differences in captures across plots would accurately reflect relative abundance regardless of the actual number of recaptures. If our observations were not a result of differences in relative abundance, then the observed results would be caused by a greater amount of recaptures within stilt grass patches. This would suggest greater activity within stilt grass (as opposed to greater abundance) since animals need to be moving in order to fall into a pitfall trap. Second, this study was limited to a single preserve, so the observed patterns may not occur in other areas invaded by stilt grass. We particularly stress the fact that this study compared stilt grass-invaded patches to noninvaded areas of virtually no other herbaceous cover, native or exotic. In this preserve, this contrast is relevant because deer browsing pressure is so intense that there is no other significant herbaceous cover of any kind in the hardwood forest, though there are some areas of substantial fern cover in the interior old growth hemlock stands. It is certainly likely that stilt grass would be less suitable compared to an intact herbaceous community. However, such pristine locations are becoming increasingly rare in the suburban northeast.
Our data show that these anuran species (particularly pickerel and wood frogs) select conditions that at least correspond to areas invaded by stilt grass. In addition, there is evidence that stilt grass may even maintain or improve local conditions for two species by retaining soil moisture, as compared to areas devoid of native herbaceous cover. The apparent importance of stilt grass presence separate from any associated microclimate variable in our models also indicates that stilt grass may maintain habitat in ways not yet investigated. These patterns should be taken into account by managers when making the decision whether to remove stilt grass, and has strong implications regarding removal method (e.g., spraying vs. pulling). Spraying stilt grass may indirectly harm amphibian species by removing important habitat in addition to any direct toxic effects. Finally, the use of a nonnative species as habitat by native wildlife in a system with degraded native vegetation raises important questions regarding the traditional "extermination is always best" policy that many managers have with regard to exotic plants. From a practical perspective, these data indicate that an exotic species can provide stable alternate habitat until native vegetation can recolonize or be restored. In the MRGP, for example, restoration of native plants would be impractical until deer abundances are lowered. Managers would, of course, prefer to remove all invasive species and fully restore important native communities but this is almost never possible. Given limited time and funds managers must prioritize their conservation goals.
Future research should work to identify which invasive species have beneficial or mitigating characteristics (and why) and which specific wildlife species are able to use nonnative habitat. Regarding the latter, while three common amphibian species appear to readily use stilt grass, the same may not be true of rarer, less generalist species of which there may be particular conservation concern. Specific management policy should obviously be decided on a site-by-site basis, though in general managers should probably continue to limit the spread of "useable" invasives. However, there may be selected invasions that could be left to provide surrogate habitat until native restoration is completed, particularly if removal would be excessively expensive or destructive.
Acknowledgements
This research was funded by a grant from the Dorr Foundation and the A.E. Charitable foundation. The authors wish to thank Dr. Robert Rockwell and Dr. John Tirpak for their assistance in analysis and interpretation, the MRGP Board of Trustees, and Peter Snell for his assistance in the field.