Spartina alterniflora and Phragmites australis as Habitat for the Ribbed Mussel, Geukensia demissa, in Saw Mill Creek of New Jersey's Hackensack Meadowlands
by Marion McClary Jr.
Fairleigh Dickinson University, School of Natural Sciences, 1000 River Road, H-DH4-03, Teaneck, NJ 07666
Abstract
In areas where the cordgrass Spartina alterniflora and the invasive common reed, Phragmites australis, coexist, P. australis is often regarded as the salt-marsh grass less populated by fauna. Although it is known that the ribbed mussel, Geukensia demissa, utilizes S. alterniflora as habitat, it was not known whether S. alterniflora is a preferred habitat for the mussel when both the cordgrass and P. australis occupy an area. To determine this, I calculated the mean number of G. demissa in four replicate quadrats near P. australis and four replicate quadrats near S. alterniflora in Saw Mill Creek of the Hackensack Meadowlands, New Jersey, in March, June, and October 2002 and June 2003. Ribbed mussels were significantly more numerous near P. australis than near S. alterniflora in March 2002 and tended to be somewhat more numerous near P. australis on the other three sampling dates, suggesting that P. australis provides as good, if not better, habitat for G. demissa as S. alterniflora. Since Saw Mill Creek is a unique ecosystem due to human intervention, the results of this study should not be assumed to be true in areas where S. alterniflora and P. australis coexist and similar human influence is absent.
Key words: common reed; cordgrass; Geukensia; habitat; Phragmites; ribbed mussel; Spartina
Introduction
There has been much concern about the effects the invasion of the common reed, Phragmites australis, has on salt marshes that have been dominated by the cordgrass Spartina alterniflora. The common reed flattens the marsh surface, lowers the water table and the salinity of the soil (Windham & Lathrop, 1999), and converts mosaics of vegetation into dense monotypic stands (Marks, Lapin & Randall, 1994; Chambers, Meyerson & Saltonstall, 1999; Galatowitsch, Anderson & Ascher, 1999; Windham & Lathrop, 1999; Rice, Rooth & Stevenson, 2000). It may also increase sedimentation (Buttery & Lambert, 1965) and build up the marsh plain (Windham, 1995).
These actions, and possibly others, may be altering habitat for salt-marsh plants and animals. Marks, Lapin, and Randall (1993) found that several rare and endangered plant populations were threatened by P. australis invasion. Benoit and Askins (1999) found that the biodiversity of flowering plants and birds was reduced in P. australis–dominated marshes. Phragmites australis is often regarded as a salt-marsh grass that is less populated by fauna than S. alterniflora. Roman, Niering, and Warren (1984) found that waterfowl usage was substantially reduced in marshes invaded by P. australis. Rozas and Odum (1987); Kneib (1994); Kneib and Wagner (1994); Able and Hagan (2000, 2003); Raichel, Able, and Hartman (2003); and Able, Hagan, and Brown (2003) reported that larval and juvenile fish usage of the marsh surface was affected. Angradi, Hagan, and Able (2001) found that the density of benthic macroinvertebrates was lower in P. australis than in S. alterniflora in August and October.
Concern about habitat alteration has often led to the physical removal of P. australis and the planting of S. alterniflora in its place (Marks et al., 1994; Weinstein, Balletto, Teal & Ludwig, 1997; Weinstein, Phillip & Goodwin, 2000; Weinstein, Teal, Balletto & Strait, 2001). As a restoration solution, this has been costly and sometimes less than successful (Melvin-Stefani & Webb-James, 1998).
Moreover, there is evidence supporting the view that P. australis does not have a deleterious effect on the ability of marshes to function as habitat for fauna. Fell et al. (1998) and Warren et al. (2001) reported that fish foraging on invertebrates and the abundance of invertebrates was not affected by the expansion of P. australis. Others have found that fish species composition was also not affected by common reed invasion (Able and Hagen, 2000; Meyer, Johnson & Gill, 2001). Wainright, Weinstein, Able, and Currin (2000) reported that P. australis may contribute to the food chain in marsh systems.
Offering weight to both sides of the issue, Talley and Levin (2001) reported that invading P. australis stands had more podurid insects, sabellid polychaetes, and peracarid crustaceans but fewer epifaunal gastropods, arachnids, midges, and tubificid and enchytraeid oligochaetes than uninvaded stands. Their findings varied with season, site, and salinity.
It is well known that the ribbed mussel, Geukensia demissa, utilizes S. alterniflora as habitat (Kuenzler, 1961a,b; Castagna & Chanley, 1973; Stiven & Kuenzler, 1979; Jordan & Valiela, 1982; Bertness, 1984; Bertness & Grosholz, 1985). There is evidence that ribbed mussels benefit S. alterniflora by attaching to the plant's root mat and strengthening it against physical disturbance and erosion. The mussels' filter-feeding activities may also oxygenate the sediments and provide them with nitrogenous wastes and minerals (Jordan & Valiela, 1982), contributing in turn to an increase in the above- and below-ground biomass of S. alterniflora (Bertness, 1984).
Though the associations between S. alterniflora and G. demissa are known, information about possible associations between P. australis and G. demissa is lacking. The purpose of this study was to determine if one marsh grass is more densely populated by G. demissa when S. alterniflora and P. australis coexist.
Materials and Methods
The study was conducted in the Hackensack Meadowlands of New Jersey, west of the Hackensack River, in a tidal tributary of Saw Mill Creek, itself a tributary of the Hackensack River (40°46'N, 74°06'W). The west side of the tidal tributary is dominated by P. australis, and the east side is dominated by native S. alterniflora. Phragmites australis was planted in the Meadowlands to stabilize the banks of mosquito ditches at a time when the plant was not considered invasive (Headlee, 1945). Dikes, tidal restrictions (Roman et al., 1984), drainage or mosquito ditches (Bart, 1997; Bart & Hartman, 2000), and construction creating higher ground such as roads (Bart, 1997; Keller, 2000; Ailstock, Norman & Bushmann, 2001) have been found to be associated with invasions of P. australis. Dikes, roads (e.g., the New Jersey Turnpike), and railroads surround Saw Mill Creek, and it is possible that such construction may have aided the expansion of P. australis at the study site. Prior to this construction, it is possible that the study site was dominated by S. alterniflora. The presence of both P. australis and S. alterniflora in Saw Mill Creek may be the result of the failure of dikes during storms, as this would have allowed the tide to come in again and the saltwater species S. alterniflora to recolonize. Remnants of these dikes can still be seen at the mouth of Saw Mill Creek where it drains into the Hackensack River. Their failure has also allowed tidal flushing of P. australis stands, and this, along with salinity changes, may be responsible for the rarely seen presence of G. demissa near P. australis.
Because of the sparse population of G. demissa on either side of the tidal tributary, possibly due to low salinity, quadrats were not located along a transect line. The location of each quadrat was determined by the presence of at least one mussel, and so was not random. This "chosen meter" method included nearly every mussel that was present at the site. Each quadrat measured one square meter; the number of G. demissa in one square meter of marsh was sampled by counting the number of mussels found within each quadrat. Four replicate quadrats, which did not overlap, were surveyed around P. australis, along with another four replicate quadrats around S. alterniflora, in March, June, and October 2002 and June 2003. The same eight quadrats were not repeatedly sampled; however, the area where they were made, consisting of a sparse population of mussels and including nearly every mussel at the site, was sampled repeatedly. The mean number of G. demissa in four replicates of the chosen meters around P. australis and four replicates of the chosen meters around S. alterniflora was calculated. A one-way ANOVA and a Dunnett's Multiple Comparison Test were used to determine whether the means were significantly different. The means were considered to be significantly different when p < 0.05. The sizes of the mussels around P. australis and S. alterniflora were not measured.
Results and Discussion
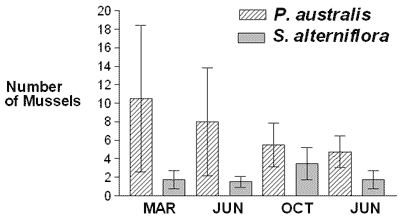
Geukensia demissa was significantly more numerous near P. australis than near S. alterniflora in March 2002 and tended to be somewhat more numerous near P. australis on the other three sampling dates, suggesting that P. australis may provide as good, if not better, habitat for G. demissa as S. alterniflora (Figure 1). These findings, from a habitat perspective, are consistent with those of Fell et al. (1998), Able and Hagen (2000), Meyer et al. (2001), and Warren et al. (2001), as outlined above.
They are not, however, consistent with the findings of other researchers, also outlined above (Roman et al., 1984; Rozas & Odum, 1987; Kneib, 1994; Kneib &Wagner, 1994; Benoit & Askins, 1999; Able & Hagan, 2000, 2003; Angradi et al., 2001; Talley & Levin, 2001; Raichel et al., 2003; Able et al., 2003). Neither are they consistent with Posey, Alphin, Meyer & Johnson (2003), who reported a slightly higher abundance of fauna in S. alterniflora marshes than in P. australis marshes.
There could be several reasons for the inconsistency. The most basic one is the difference between the species and sites studied. In this study, habitat usage was evaluated using a semisessile species, G. demissa. Mussels are an excellent species to use in habitat studies because they generally don't move very far from the habitat where they settle, and when they do, their rate of movement is slow. Animals such as waterfowl and fish are more difficult to use when evaluating habitats because they migrate. If their migratory patterns are not known or accounted for when sampling, this can have a profound effect on the study results. Other reasons for the inconsistency include the presence of shallow pools around S. alterniflora and the lack of them around P. australis, possible differences in food availability, and differences in stem density and/or canopy thickness (Fell, Warren, Light, Rawson & Fairley, 2003). P. australis populations often occur in large dense stands with 100% cover; S. alterniflora populations are patchy. It is possible that variations in the spatial dynamics of the population of each species from one site to the next are responsible for the variable results on the effects of P. australis and S. alterniflora as habitat for animals.
It is likely that after March 2002, there was more predation and/or other mortality of G. demissa near P. australis; and between June and October 2002, there may have been more recruitment of G. demissa near S. alterniflora. The extent of mortality and recruitment at each site is currently being studied by marking individual mussels. If recruitment of G. demissa to P. australis and S. alterniflora is different, future studies will determine whether this difference is due to habitat selection by larval G. demissa or to hydrodynamic factors. In other studies currently being conducted, half the mussel populations are being caged to gain additional information on predation of G. demissa near S. alterniflora and near P. australis.
The construction of mosquito ditches, roads, railroads, dikes, and their failure in storms make Saw Mill Creek of the Hackensack Meadowlands a unique ecosystem where both S. alterniflora and P. australis coexist. Although the results of this study indicate that P. australis may provide comparable, if not better, habitat for G. demissa than S. alterniflora, the results should not be assumed to be true in areas where S. alterniflora and P. australis coexist but the kind of human intervention that exists in Saw Mill Creek is absent. Future studies will investigate such areas and determine whether G. demissa is also present in other parts of the Meadowlands that are dominated by P. australis.
Acknowledgments
Brett A. Bragin, Edward Konsevick, Jeffery Misuik, Joseph Sarnoski, and Craig Woolcott of the Meadowlands Environmental Research Institute provided staff support in the field. Dr. Francisco Artigas, Dr. Kirk Barrett, James Cramer, Leonard Houston, and Dr. Erik Kiviat of the Meadowlands Symposium Organizing Committee invited this paper for publication. Two anonymous reviewers; Niall Dunne, the associate editor of Urban Habitats; and Gerry Moore, the science editor of Urban Habitats, greatly improved the manuscript with their comments and suggestions and recommended its publication. The New Jersey Sea Grant College Program funded the follow-up work (R/D-2003-3).